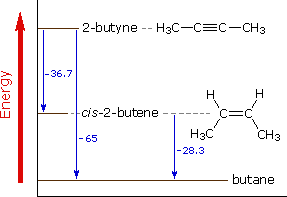
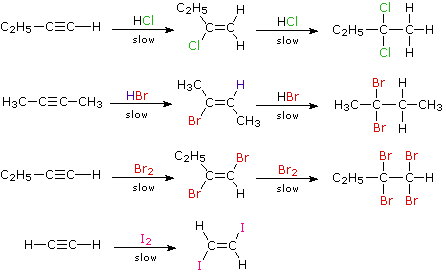
Simple addition of bonds. True sp-hybridization on the carbon triple bond keeps the pair of electrons closer to the nucleus.
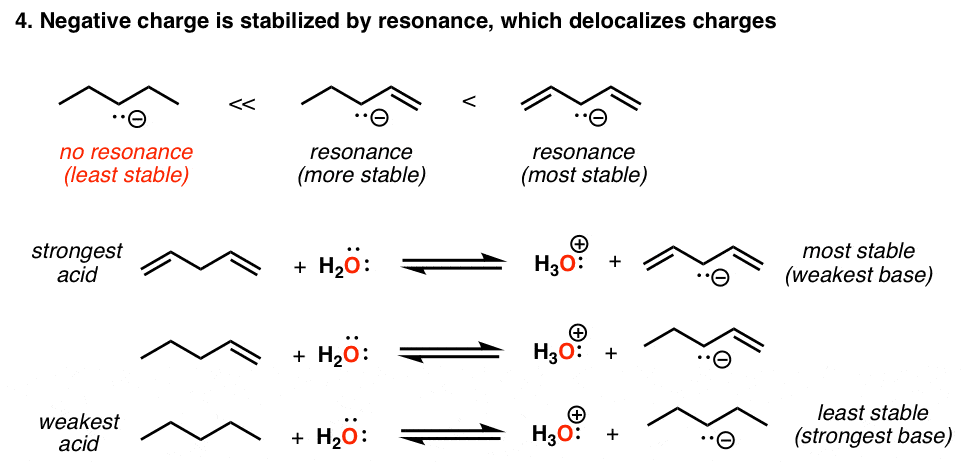
Acidity Trends In Organic Chemistry Master Organic Chemistry
Alternatively double bonds are hydroxylated by peracids while triple bonds remain unchanged 41.
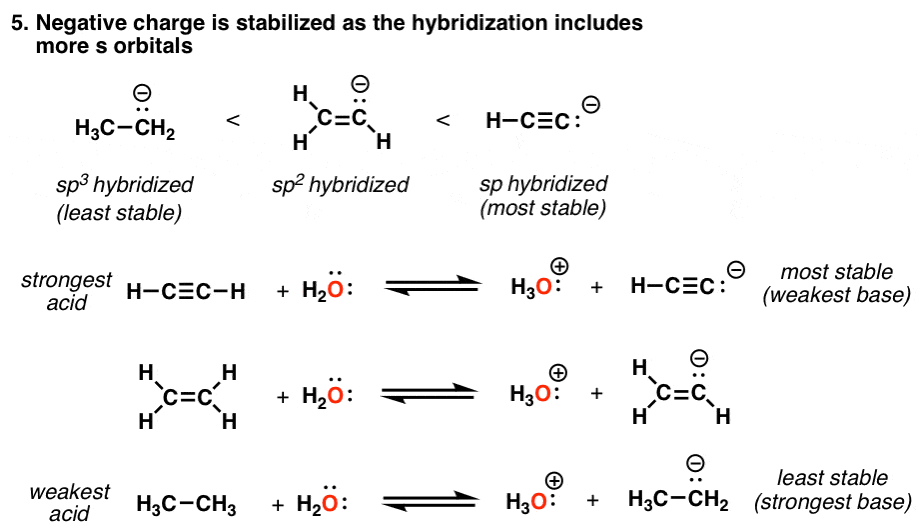
. I know that triple bonds are more acidic than double bonds and double bonds are more acidic than singlebut why. As a result of it s-character in triple bond is more about 50 as compare to double bond about 333 so acidity of triple bond is more as compare to double bond. If you think about it a triple bond has one sigma and two pi bonds.
N has higher electronegativity than C so I figured the first compound would be the correct answer but it wasnt. Double and triple bonds. The main difference between single double and triple bond is the number of shared electrons.
Only the lower locant for a multiple bond is cited except when the numerical difference between the. Bond order can be defined as the number of electron bonding pairs present between two atoms. If there is a choice choose the chain.
The semihydrogenation of a carbon-carbon triple bond can be accomplished in molecules containing double bonds whether isolated or conjugated with one another. Fatty acids with one double bond are the most prevalent in the human body comprising about half of the total. This video shows chemical bonds inside human body respiration breathing.
Press question mark to learn the rest of the keyboard shortcuts. Obviously triple bond because it is slightly acidic and in case of smaller alkynes like acetylene acidic nature increases due to terminating hydrogen but in case of double bonds not that much. The idea is that even though the bond itself double or triple is stronger than a single bond as a whole we must consider the components of a double or triple bond.
Here is my understanding of the rules. A proton on a carbon triple bond will be more stable than a proton on a carbon double bond. The atoms with triple valency are pnictogen or the nitrogen family.
You may have heard that. I also am not sure why triple bonds are more acidic than double and double are more acidic than single. I thought that the more carbon atoms the higher worse the pka.
I am pretty sure triple bonds are the strongest then double then single. Oxygen atoms can form double bonds and nitrogen atoms can form triple bonds to ma. Double and triple bonds one sigma and one pi or one sigma and two pi are more stable than a single bond which consists of just one sigma bond.
A single bond has a bond order of one a double bond has a bond order of two and similarly. When we add oxygen to the formula we assess the degree of unsaturation. If a choice remains preference for low locants is given to the double bonds.
N 2 C 2 H 2 etc. Im studying for my orgo test and Im confused as to why this triple bond is more acidic. If the shared number is one pair of electrons the bond will be a single bond.
In triple bond sp hybridisation and in double bond Sp2 hybridisation is present. There are normally 4 e- regions either bonds or lone pairs around the C or N. And so for a given formula each degree of unsaturation corresponds to a double bond OR a ring.
One sigma bond and two pi bonds make. This impression is not necessarily true. Press J to jump to the feed.
Choose the longest continuous chain of carbon atoms whether or not it contains multiple bonds. Triple bond is denoted by three dash joining the atoms. In this way double and triple bonds in a single fatty acid can be differentiated 575.
In addition to single bonds atoms can form double or triple bonds. Fatty acids with two or more double bonds occur in lesser quantities but are. All Answers 4 This is because double and triple bonds are formed by lateral overlapping of p orbitals whereas single bonds are formed by head on collision of orbitals so.
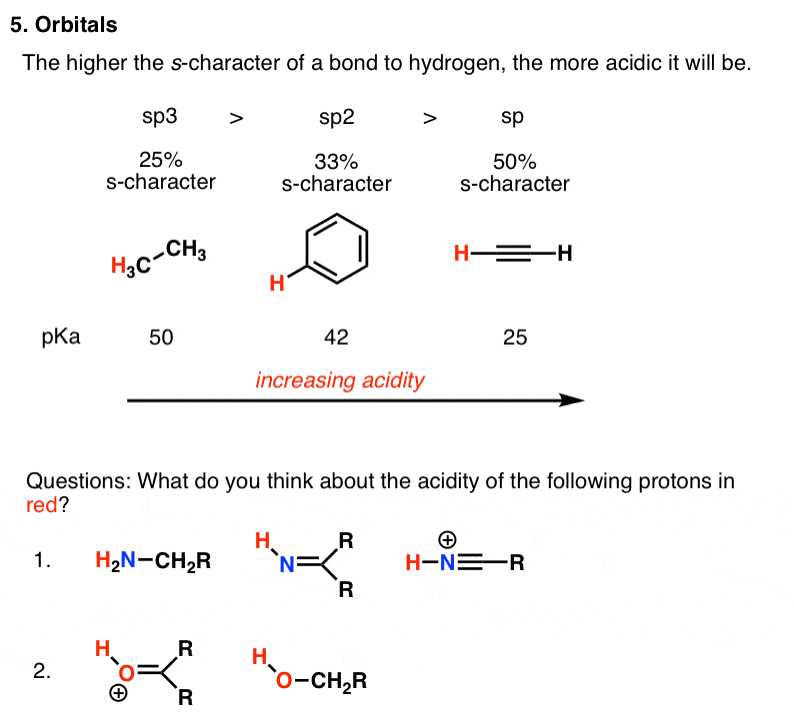
5 Key Factors That Influence Acidity In Organic Chemistry

Acid Base Which Will Be The Most Acidic Hydrogen In The Following Organic Compounds Chemistry Stack Exchange
A Double Bond Is Stronger Than A Triple Bond Why Quora

Acidity Trends In Organic Chemistry Master Organic Chemistry
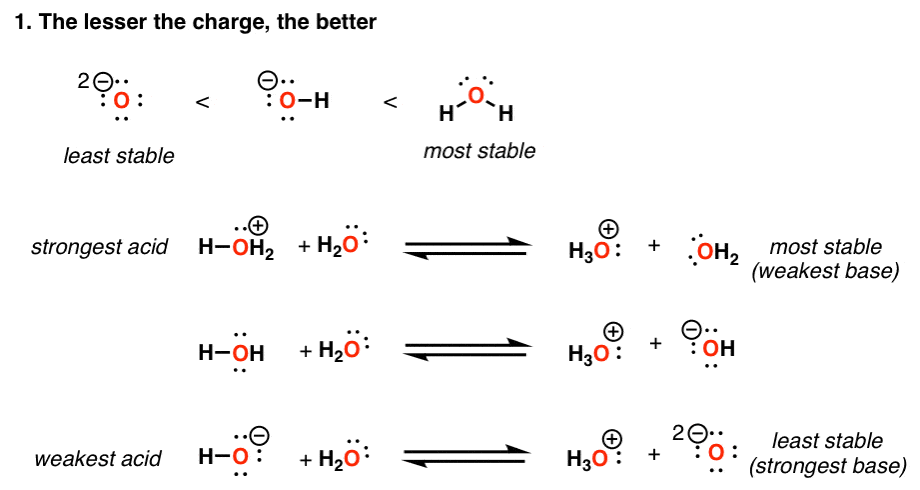
Acidity Trends In Organic Chemistry Master Organic Chemistry
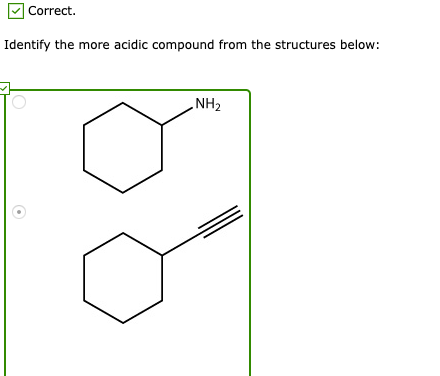
Solved Why Are Triple Bonds More Acidic Than Double Chegg Com
Is Tautomerism Possible In The Triple Bond Of Carbon Quora
Why Is The Acidity Of A Triple Bond Greater Than A Double Bond Quora

Alkynes Alkynes Are Molecules That Incorporate A C C Triple Bond Ppt Download
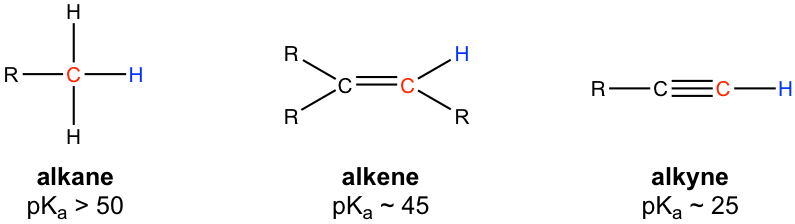
10 8 Alkynes Organic Chemistry I
Why Is The Acidity Of A Triple Bond Greater Than A Double Bond Quora

Triple Bond An Overview Sciencedirect Topics
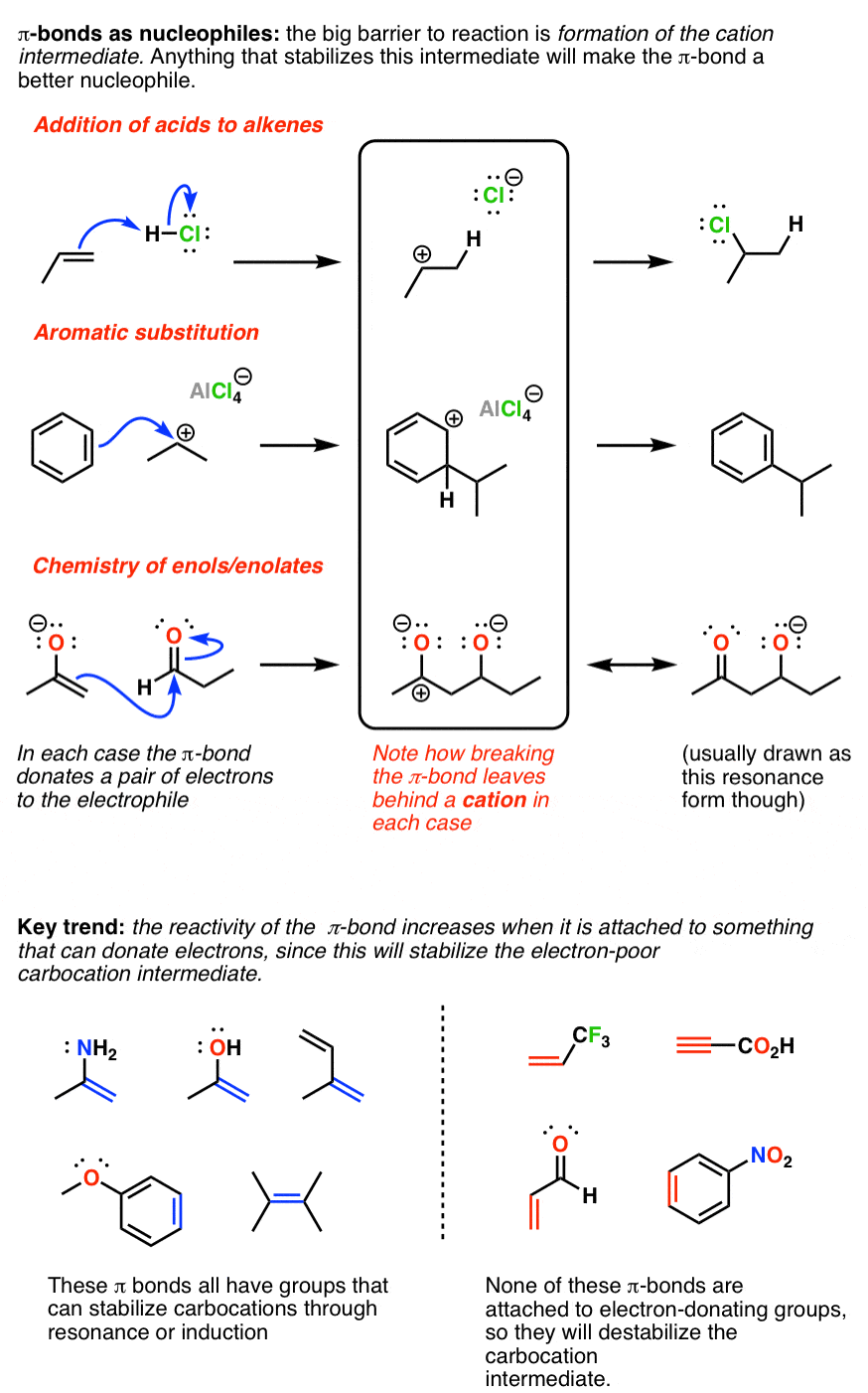
The Three Classes Of Nucleophiles Master Organic Chemistry
Why Is The Acidity Of A Triple Bond Greater Than A Double Bond Quora

Triple Bond An Overview Sciencedirect Topics